About

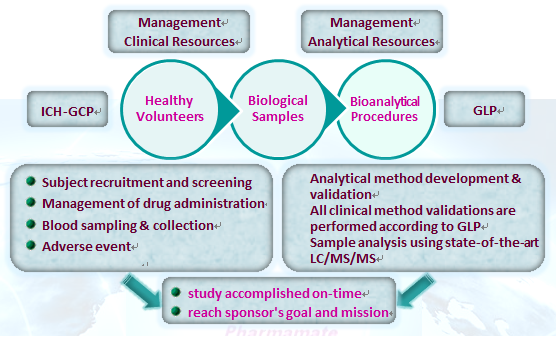
Rosetta a Contract Research Organization for drug development, was incorporated in October 2002 and located in New Taipei City, Taiwan. We offering a high quality and cost-effectiveness service for PK, BA/BE, Phase I study, Bioanalytical, Bridging Study etc.. In highly trained staff and precision instruments, we are capable to provide in-depth support to help our client in drug development.
leading innovation
Performance / Experiences
News / Certification
Info
- Bioanalytical Laboratory has been inspected by the TFDA (Taiwan Food and Drug Administration, Ministry of Health and Welfare of Republic of China) in Sep,2013
- Establish Animal Facility in Oct,2016
- Bioanalytical Laboratory and Clinical site have been inspected 3rd by the NPRA (National Pharmaceutical Regulatory Agency, Ministry of Health Malaysia) in Oct,2024

